Cerium Glows Yellow: Chemists Discover How to Control Luminescence of Rare Earth Elements
.jpg)
Researchers at HSE University and the Institute of Petrochemical Synthesis of the Russian Academy of Sciences have discovered a way to control both the colour and brightness of the glow emitted by rare earth elements. Their luminescence is generally predictable—for example, cerium typically emits light in the ultraviolet range. However, the scientists have demonstrated that this can be altered. They created a chemical environment in which a cerium ion began to emit a yellow glow. The findings could contribute to the development of new light sources, displays, and lasers. The study has been published in Optical Materials.
Rare earth elements are used in microelectronics, LEDs, and fluorescent materials because of their ability to emit light in precisely defined colours. This depends on how their electrons behave when absorbing and releasing energy.
When an atom absorbs energy—such as from light or an electric current—one of its electrons can be excited to a higher energy level. However, this excited state is unstable, and after a short time, the electron returns to its original level, releasing the excess energy as light. This process is known as luminescence.
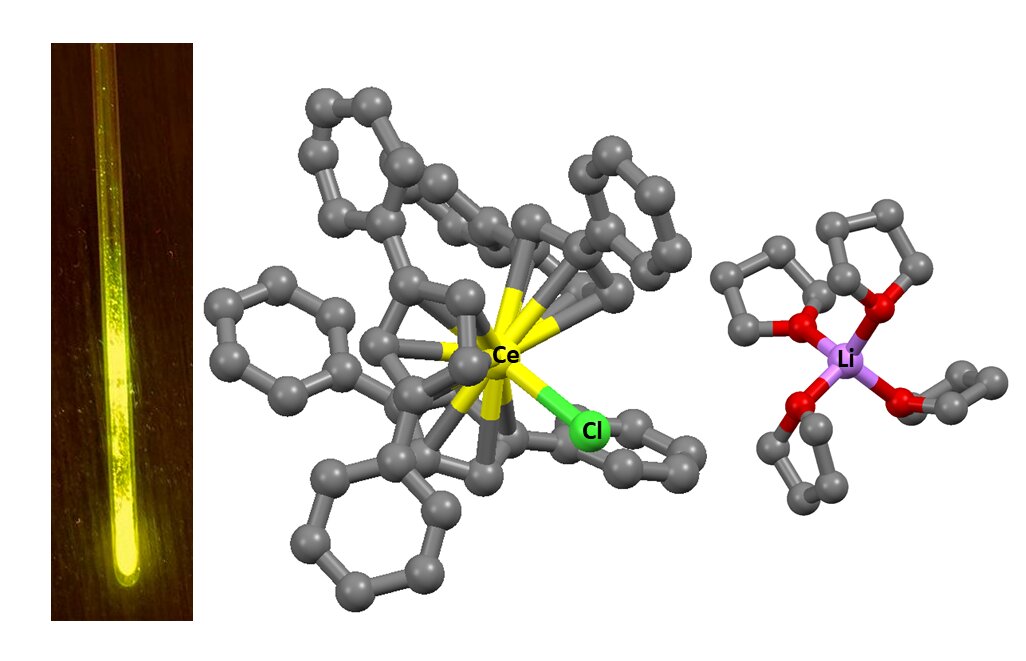
In rare earth elements, the glow results from electron transitions between 4f orbitals—regions around the atomic nucleus where electrons can reside. Typically, the energy of these transitions is fixed, meaning the colour of the glow remains constant: cerium emits invisible ultraviolet light, while terbium emits green. The 4f orbitals are situated deep within the atom and interact minimally with the surrounding environment. In contrast, the 5d orbitals are sensitive to external influences but generally do not contribute to the luminescence of lanthanides due to their excessively high energy.
However, scientists from HSE University and the Institute of Petrochemical Synthesis of the Russian Academy of Sciences have demonstrated that the colour of the radiation can be altered by adjusting the chemical environment of the metals. They synthesised cerium, praseodymium, and terbium complexes using organic ligands—molecules that surround metal ions. These ligands shape the geometry of the complex and influence its properties. In all cases, three cyclopentadienyl anions were symmetrically arranged around the metal. These anions consist of regular pentagons of carbon atoms, to which large organic fragments are attached, providing the required structure for the complex. This environment generates a specific electrostatic field around the ion, which alters the energy of the 5d orbitals and, consequently, affects the luminescence spectrum.

Daniil Bardonov
'Previously, a change in the colour of the glow had been observed, but the underlying mechanism was not understood. Now, in collaboration with our physicist colleagues, we have been able to understand the mechanism behind this effect. We deliberately designed compounds with an electronic structure that is atypical for lanthanides. Rather than focusing on a single example, we synthesised a series of compounds from cerium to terbium to observe how their properties change and to identify common patterns,' comments Daniil Bardonov, a master's student at the HSE Faculty of Chemistry.
In conventional compounds, cerium emits ultraviolet light with wavelengths between 300 and 400 nanometres. In the new complexes, its emission shifted to the red range, reaching up to 655 nanometres. This indicates that the energy gap between the 4f and 5d levels has decreased. A similar rearrangement of electronic levels was observed in the other lanthanides studied, also resulting in changes to their luminescence.

Dmitrii Roitershtein
'To understand how this process works, it’s important to first grasp the mechanism of energy transfer. Typically, a ligand molecule absorbs ultraviolet light, enters an excited state, and then transfers this energy to the metal atom, causing it to emit light,' explains Dmitrii Roitershtein, Academic Supervisor of the Chemistry of Molecular Systems and Materials Programme and co-author of the paper. 'However, in the new compounds, the process occurred differently: energy was transferred not directly to the 4f electrons, but via an intermediate 5d state.'
The researchers believe that being able to predict the luminescence spectrum will make it possible to design materials with desired properties more efficiently by eliminating the need for time-consuming trial and error. This could facilitate the creation of new and advanced light sources.
'We were able to demonstrate exactly how the environment of an atom influences its electronic transitions and lanthanide luminescence,' says Fyodor Chernenkiy, bachelor's student at the HSE Faculty of Chemistry. 'We can now intentionally select the structure of compounds to control luminescence and produce materials with specific optical properties.'
See also:
Scientists Uncover Why Consumers Are Reluctant to Pay for Sugar-Free Products
Researchers at the HSE Institute for Cognitive Neuroscience have investigated how 'sugar-free' labelling affects consumers’ willingness to pay for such products. It was found that the label has little impact on the products’ appeal due to a trade-off between sweetness and healthiness: on the one hand, the label can deter consumers by implying an inferior taste, while on the other, it signals potential health benefits. The study findings have been published in Frontiers in Nutrition.
HSE Psycholinguists Launch Digital Tool to Spot Dyslexia in Children
Specialists from HSE University's Centre for Language and Brain have introduced LexiMetr, a new digital tool for diagnosing dyslexia in primary school students. This is the first standardised application in Russia that enables fast and reliable assessment of children’s reading skills to identify dyslexia or the risk of developing it. The application is available on the RuStore platform and runs on Android tablets.
Physicists Propose New Mechanism to Enhance Superconductivity with 'Quantum Glue'
A team of researchers, including scientists from HSE MIEM, has demonstrated that defects in a material can enhance, rather than hinder, superconductivity. This occurs through interaction between defective and cleaner regions, which creates a 'quantum glue'—a uniform component that binds distinct superconducting regions into a single network. Calculations confirm that this mechanism could aid in developing superconductors that operate at higher temperatures. The study has been published in Communications Physics.
Neural Network Trained to Predict Crises in Russian Stock Market
Economists from HSE University have developed a neural network model that can predict the onset of a short-term stock market crisis with over 83% accuracy, one day in advance. The model performs well even on complex, imbalanced data and incorporates not only economic indicators but also investor sentiment. The paper by Tamara Teplova, Maksim Fayzulin, and Aleksei Kurkin from the Centre for Financial Research and Data Analytics at the HSE Faculty of Economic Sciences has been published in Socio-Economic Planning Sciences.
Larger Groups of Students Use AI More Effectively in Learning
Researchers at the Institute of Education and the Faculty of Economic Sciences at HSE University have studied what factors determine the success of student group projects when they are completed with the help of artificial intelligence (AI). Their findings suggest that, in addition to the knowledge level of the team members, the size of the group also plays a significant role—the larger it is, the more efficient the process becomes. The study was published in Innovations in Education and Teaching International.
New Models for Studying Diseases: From Petri Dishes to Organs-on-a-Chip
Biologists from HSE University, in collaboration with researchers from the Kulakov National Medical Research Centre for Obstetrics, Gynecology, and Perinatology, have used advanced microfluidic technologies to study preeclampsia—one of the most dangerous pregnancy complications, posing serious risks to the life and health of both mother and child. In a paper published in BioChip Journal, the researchers review modern cellular models—including advanced placenta-on-a-chip technologies—that offer deeper insights into the mechanisms of the disorder and support the development of effective treatments.
Using Two Cryptocurrencies Enhances Volatility Forecasting
Researchers from the HSE Faculty of Economic Sciences have found that Bitcoin price volatility can be effectively predicted using Ethereum, the second-most popular cryptocurrency. Incorporating Ethereum into a predictive model reduces the forecast error to 23%, outperforming neural networks and other complex algorithms. The article has been published in Applied Econometrics.
Administrative Staff Are Crucial to University Efficiency—But Only in Teaching-Oriented Institutions
An international team of researchers, including scholars from HSE University, has analysed how the number of non-academic staff affects a university’s performance. The study found that the outcome depends on the institution’s profile: in research universities, the share of administrative and support staff has no effect on efficiency, whereas in teaching-oriented universities, there is a positive correlation. The findings have been published in Applied Economics.
Physicists at HSE University Reveal How Vortices Behave in Two-Dimensional Turbulence
Researchers from the Landau Institute for Theoretical Physics of the Russian Academy of Sciences and the HSE University's Faculty of Physics have discovered how external forces affect the behaviour of turbulent flows. The scientists showed that even a small external torque can stabilise the system and extend the lifetime of large vortices. These findings may improve the accuracy of models of atmospheric and oceanic circulation. The paper has been published in Physics of Fluids.
Solvent Instead of Toxic Reagents: Chemists Develop Environmentally Friendly Method for Synthesising Aniline Derivatives
An international team of researchers, including chemists from HSE University and the A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences (INEOS RAS), has developed a new method for synthesising aniline derivatives—compounds widely used in the production of medicines, dyes, and electronic materials. Instead of relying on toxic and expensive reagents, they proposed using tetrahydrofuran, which can be derived from renewable raw materials. The reaction was carried out in the presence of readily available cobalt salts and syngas. This approach reduces hazardous waste and simplifies the production process, making it more environmentally friendly. The study has been published in ChemSusChem.


